Which Best Describes Rutherfords Model of the Atom
There are no electrons in the Rutherford Model. Berdasarkan model atom berikut yang merupakan model atom dalton adalah.
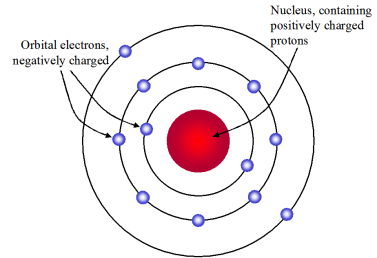
Rutherford Model Which Best Describes Rutherford S Model Chemistry
He believed that electrons moved around the nucleus in.

. According to the Rutherford model an atom is largely empty space comprising electrons that are negatively charged surrounding. Which of the following statements BEST describes the location of electrons in Rutherfords model of the atom. 9 Which statement best describes the part of the atom that is shown by the arrow.
Which statement best summarizes the importance of ernest rutherfords gold foil experiment. According to the Rutherford atomic model. The atom cannot be divided into smaller particles.
This answer has been confirmed as correct and helpful. Correct answer - Which best describes rutherfords model of the atom. The modern-day quantum model of the atom is better than john daltons model because it.
Which best describes rutherfords model of the atom. Which best describes the current model of the atom. Which best describes the current model of an atom.
Rutherfords model of the atom consisted of a positively charged center known as NUCLEUS which also contained most of the atoms mass. The current model of an atom is best described by the Solar System. According to Rutherford model of the atom.
Added 4172021 20039 PM. He called this region of the atom as a nucleus. The electrons are inside the nucleus.
As there was very less deflection of alpha particles so he concluded that most of the space was empty in an atom. 12 How are the elements arranged in the periodic table. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom.
According to rutherfords model of the atom electrons behave like. The electrons are located within the positive material of the nucleus. Which statement best describes the quantum mechanical modern model of the atom.
10 How are electrons arranged in an atom. 1 Atoms have their charge concentrated in a very small nucleus. Which best describes Rutherford and model of the atom.
14 How do we know the structure of an. Which statement describes one feature of Rutherfords model of the atom. With these measurements he came to the conclusion that almost the entire mass of the atom had to be in a very small space that I call a nucleus in.
The electrons are outside the nucleus. 11 Which of the following statements correctly defines the atomic mass of an element. He also concluded that positive charge contains very less amount of space in an atom as very few articles were deflected from their path.
Rutherford concluded the following points after the observation. Around the nucleus orbited the. The statements which describe Rutherfords model of the atom are as follows.
The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. 13 What atomic model accurately explains the structure of an atom. 2 Major space in an atom is empty.
Electrons are both inside and outside of the nucleus. The atom is mostly empty space. 2 Major space in an atom is empty.
Best describes how the current scientific model of the atom was developed. Which conclusion could be made from ernest rutherfords gold foil experiment. An electron with n 2 is at higher energy than an electron with n 1.
This model of an atom was developed by Ernest. It is the part of the atom with the greatest mass. Rutherford discovered the proton and supposed.
According to the new. 4 An atom is electrically neutral. An atomic model of Rutherford doesnt exist.
Electrons orbit around the center of the atom. Biology 20092019 2030 Dhejxbdbbxbdbbx Which best describes rutherfords model of the atom. Rutherfords experiment was to bombard a gold foil with alpha particles finding that most of the particles change their direction very little some change it a lot and very few almost recede.
The quantum mechanical model of the atom Introduction to the quantum mechanical model of the atom. The Quantum-Mechanical Model of the Atom. Advertisement Answer 35 5 106.
The atom is mostly empty space. He also claimed that the electrons surrounding. Electron clouds are regions where electrons are likely to be found.
The atoms positive charge is located in the atoms nucleus. How Does Bohrs Model Of The Atom Differ From RutherfordsRutherford described the atom as consisting of a tiny positive mass surrounded by a cloud of negative electrons. The model was the result of hundreds of years of experiments.
Which of these conclusions can be drawn from rutherfords experiment. How was bohrs atomic model similar to rutherfords model. Rutherfords gold foil experiment provided evidence for which of the following.
Rutherfords model shows that an atom is mostly empty space with electrons orbiting a fixed positively charged nucleus in set predictable paths. Bohr thought that electrons orbited the nucleus in quantised orbits. 3 Atoms nucleus is surrounded by negatively charged particles called electrons.

Rutherford S Atomic Model Chemistry For Non Majors

No comments for "Which Best Describes Rutherfords Model of the Atom"
Post a Comment